General Chemistry Resources
Dr. Scott Ensign
EnsignChemistry.com
Electron-domain geometries, molecular geometries, and orbital hybridizations for a central atom containing two, three, four, five, or six electron domains.
"A" represents the central atom, "X" represents a bonded domain (note there may be one, two, or three electron pairs between A and X), and "E" represents a nonbonding electron domain (a lone pair).
| 2 |
2 |
0 |
linear |
linear |
AX2

|
sp |
BeH2,CO2, C2H2


|
180 |


|
| 2 |
1 |
1 |
linear |
linear |
AXE

|
sp |
CO, N2


|
N/A |
 |
| 3 |
3 |
0 |
trigonal planar |
trigonal planar |
AX3

|
sp2 |
BH3

|
120 |
 |
| 3 |
2 |
1 |
trigonal planar |
bent |
AX2E

|
sp2 |
SO2

|
119 |
 |
| 4 |
4 |
0 |
tetrahedral |
tetrahedral |
AX4

|
sp3 |
CH4

|
109.5 |
 |
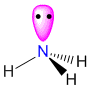
| 4 |
3 |
1 |
tetrahedral |
trigonal pyramidal |
AX3E

|
sp3 |
NH3
|
107 |
 |
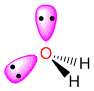
| 4 |
2 |
2 |
tetrahedral |
bent |
AX2E2

|
sp3 |
H2O
|
104 |
 |
| 5 |
5 |
0 |
trigonal bipyramidal |
trigonal bipyramidal |
AX5

|
sp3d |
PF5

|
120 and 90 |
 |
| 5 |
4 |
1 |
trigonal bipyramidal |
see saw |
AX4E

|
sp3d |
SF4

|
|
 |
| 5 |
3 |
2 |
trigonal bipyramidal |
T shaped |
AX3E2

|
sp3d |
ClF3

|
|
 |
| 5 |
2 |
3 |
trigonal bipyramidal |
linear |
AX2E3

|
sp3d |
XeF2

|
|
 |
| 6 |
6 |
0 |
octahedral |
octahedral |
AX6

|
sp3d2 |
SF6

|
180 and 90 |
 |
| 6 |
5 |
1 |
octahedral |
square pyramidal |
AX5E

|
sp3d2 |
IF5

|
|
 |
| 6 |
4 |
2 |
octahedral |
square planar |
AX4E2

|
sp3d2 |
XeF4

|
90 |
 |
© Scott Ensign, EnsignChemistry.com, all rights reserved












































