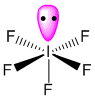
IF5 contains five bonded and one nonbonded electron domains, giving an octahedral e- domain geometry and a square pyramidal molecular geometry (AX5E). The bond angles are compressed relative to those in a perfect octahedron due to the lone pair spreading out more in space than bonded pairs. Use the check boxes to measure the bond angles for nearest and furthest atoms attached to the central atom in the equatorial plane, and for the bond angle between the single remaining axial atom, central atom, and an equatorial atom. For comparison, in a molecule with the formula AX6, the bond angles are 90 and 180 degrees for nearest and furthest atoms.
A cartoon model of the electron density of the lone pair of electrons, represented by a translucent green spheroid, can be toggled on and off. Note that the shape of the spheroid does not represent the actual electron density of the lone pair, but allows you to visualize how the presence of that lone pair dictates the molecular geometry.
Background: Black White Grey
Model type: Spacefill ball and stick stick only
Spin: Off On
Drag to turn, shift-drag vertical to zoom, shift-drag horizontal to rotate